and present a new paradigm.
- HOME
- Products
- CMF
(CranioMaxilloFacial) - Dental
- PSI
(Patient Specific Implant)
- Mesh Type(Square type)
- Plug
- Orbital
- TSI
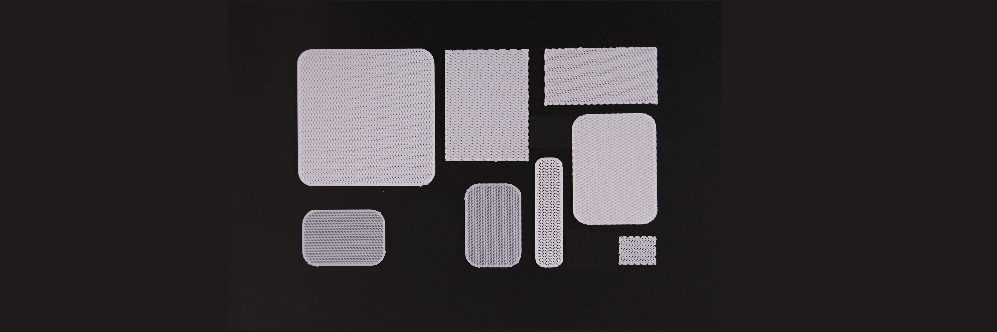
This product was fabricated using a 3D printer, and the absorbable products with a single size for each model were used for reconstruction of cranio-maxillofacial defects.
After being grafted on the body and filling the space (CMF, cranio-maxillofacial), the porous structural characteristics of the products allow the various cells normally present in the surrounding tissues to migrate into the porous scaffold. Consequently, after a certain amount of time, it promotes the generation of extracellular matrix material, such as collagen, while contributing to the reconstruction of the damaged tissue.
TnR Mesh consists of polycaprolactone (PCL). This polymer is ultimately hydrolyzed into carbon dioxide (CO2) and water (H2O) through the citric acid cycle. TnR PSI Plus is made from polycaprolactone and β-TCP(tricalcium phosphate). β-TCP plays a role in shortening the decomposition time of the product, and the hydrolysis process that takes place in the body by causing the gradual dissociation of calcium ions (Ca2+) and orthophosphate (PO43-).
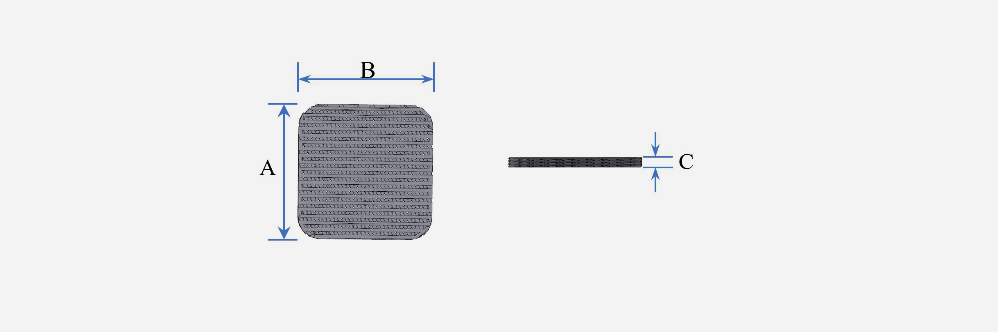
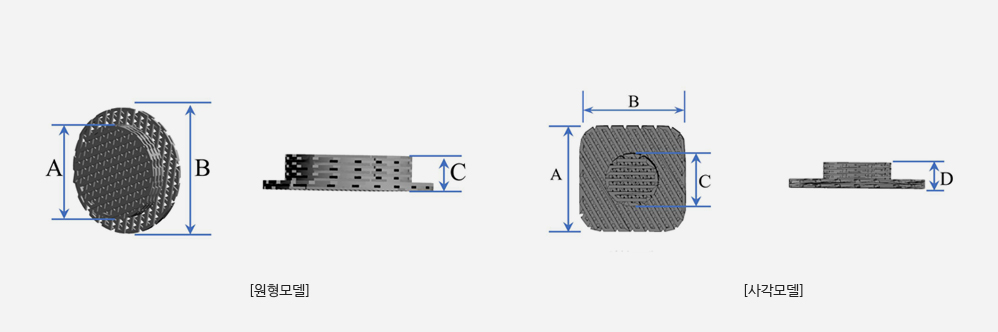
A: 5~100mm
B: 5~100mm
C: 0.15 / 0.5 / 0.8 / 1 / 1.5 / 2 / 30mm
※ A and B are in 5mm increments
A: 5~100mm
B: 5~100mm
C: 0.8 / 1 / 1.5 / 2 / 30mm
※ A and B are in 5mm increments
Size
A: 5~100mm
B: 5~100mm
C: 0.15 / 0.5 / 0.8 / 1 / 1.5 / 2 / 30mm
※ A and B are in 5mm increments
Size
A: 5~100mm
B: 5~100mm
C: 0.8 / 1 / 1.5 / 2 / 30mm
※ A and B are in 5mm increments
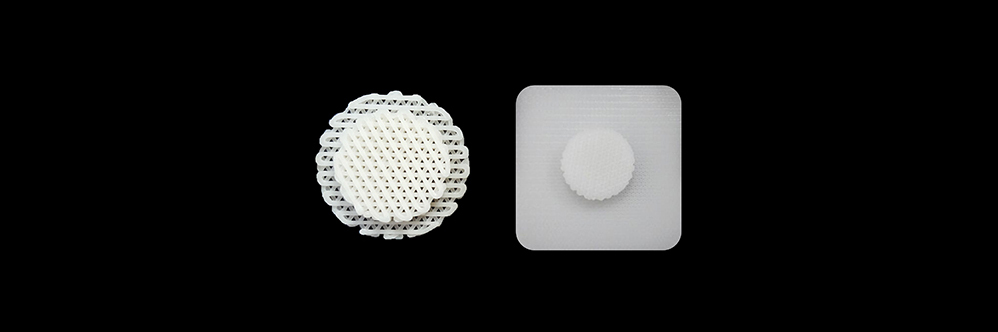
This product was fabricated using a 3D printer, and the absorbable products with a single size for each model were used for reconstruction of cranio-maxillofacial defects.
After being grafted on the body and filling the space (cranio-maxillofacial cartilage), the porous structural characteristics of the products allow the various cells normally present in the surrounding tissues to migrate into the porous scaffold. Consequently, after a certain amount of time, it promotes the generation of extracellular matrix material, such as collagen, while contributing to the reconstruction of the damaged tissue. Moreover, the polycaprolactone (PCL) polymer is ultimately hydrolyzed into carbon dioxide (CO2) and water (H2O) through a metabolic process involving the citric acid cycle.
TnR Plug PlusAfter being grafted on the body and filling the space (cranio-maxillofacial cartilage), the porous structural characteristics of the products allow the various cells normally present in the surrounding tissues to migrate into the porous scaffold. Consequently, after a certain amount of time, it promotes the generation of extracellular matrix material, such as collagen, while contributing to the reconstruction of the damaged tissue. Moreover, the polycaprolactone (PCL) polymer is ultimately hydrolyzed into carbon dioxide (CO2) and water (H2O) through a metabolic process involving the citric acid cycle. Tricalcium phosphate (TCP) plays a role in shortening the decomposition time of the product, and the hydrolysis process that takes place in the body causes gradual dissociation of calcium ions (Ca 2+) and orthophosphate (PO4 3-).
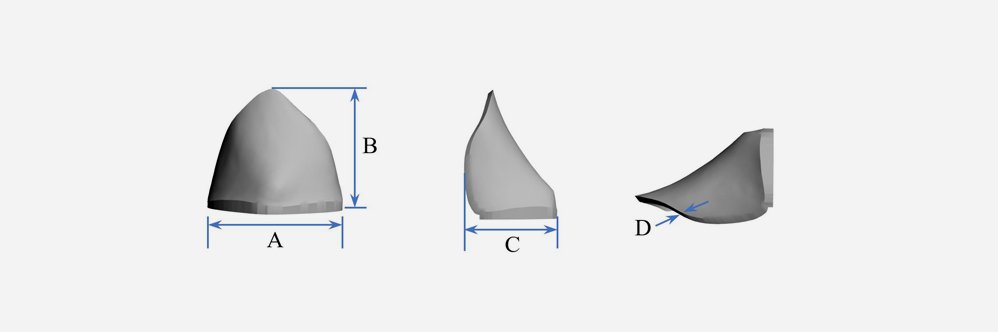
A: 5~25mm
B: 10~30mm
C: 2/5/7/10mm
※ A and B are in 5mm increments
A: 5~25mm
B: 10~30mm
C: 2/5/7/10mm
※ A and B are in 5mm increments
※ only Circular model
Size
A: 5~25mm
B: 10~30mm
C: 2/5/7/10mm
※ A and B are in 5mm increments
Size
A: 5~25mm
B: 10~30mm
C: 2/5/7/10mm
※ A and B are in 5mm increments
※ only Circular model

he product was fabricated using a 3D printer, and the absorbable products with a single size for each model were used for reconstruction of cranio-maxillofacial defects.
After being grafted on the body and filling the space (cranio-maxillofacial cartilage), the porous structural characteristics of the products allow the various cells normally present in the surrounding tissues to migrate into the porous scaffold. Consequently, after a certain amount of time, it promotes the generation of extracellular matrix material, such as collagen, while contributing to the reconstruction of the damaged tissue. Moreover, the polycaprolactone (PCL) polymer is ultimately hydrolyzed into carbon dioxide (CO2) and water (H2O) through a metabolic process involving the citric acid cycle.
TnR Mesh Plus (Square type)After being grafted on the body and filling the space (cranio-maxillofacial cartilage), the porous structural characteristics of the products allow the various cells normally present in the surrounding tissues to migrate into the porous scaffold. Consequently, after a certain amount of time, it promotes the generation of extracellular matrix material, such as collagen, while contributing to the reconstruction of the damaged tissue. Moreover, the polycaprolactone (PCL) polymer is ultimately hydrolyzed into carbon dioxide (CO2) and water (H2O) through a metabolic process involving the citric acid cycle. Tricalcium phosphate (TCP) plays a role in shortening the decomposition time of the product, and the hydrolysis process that takes place in the body causes gradual dissociation of calcium ions (Ca 2+) and orthophosphate (PO4 3-).
A: 20/30/36/40/45/50mm
B: 20/25/30/35/39/45/47mm
C: 10/14/20/25/30/35/42mm
D: 0.5/1.15/2mm
A: 20/30/36/40/45/50mm
B: 20/25/30/35/39/45/47mm
C: 10/14/20/25/30/35/42mm
D: 0.5/1.15/2mm
Size
A: 20/30/36/40/45/50mm
B: 20/25/30/35/39/45/47mm
C: 10/14/20/25/30/35/42mm
D: 0.5/1.15/2mm
Size
A: 20/30/36/40/45/50mm
B: 20/25/30/35/39/45/47mm
C: 10/14/20/25/30/35/42mm
D: 0.5/1.15/2mm
This product was fabricated using a 3D printer and the absorbable products with a single size for each model were used for reconstruction of sphenoid bone defects.
After being grafted on the body and filling the space (cranio-maxillofacial cartilage), the porous structural characteristics of the products allow the various cells normally present in the surrounding tissues to migrate into the porous scaffold. Consequently, after a certain amount of time, it promotes the generation of extracellular matrix material, such as collagen, while contributing to the reconstruction of the damaged tissue. Moreover, the polycaprolactone (PCL) polymer is ultimately hydrolyzed into carbon dioxide (CO2) and water (H2O) through a metabolic process involving the citric acid cycle.
TnR Mesh Plus TSIAfter being grafted on the body and filling the space (cranio-maxillofacial cartilage), the porous structural characteristics of the products allow the various cells normally present in the surrounding tissues to migrate into the porous scaffold. Consequently, after a certain amount of time, it promotes the generation of extracellular matrix material, such as collagen, while contributing to the reconstruction of the damaged tissue. Moreover, the polycaprolactone (PCL) polymer is ultimately hydrolyzed into carbon dioxide (CO2) and water (H2O) through a metabolic process involving the citric acid cycle. Tricalcium phosphate (TCP) plays a role in shortening the decomposition time of the product, and the hydrolysis process that takes place in the body causes gradual dissociation of calcium ions (Ca 2+) and orthophosphate (PO4 3-).
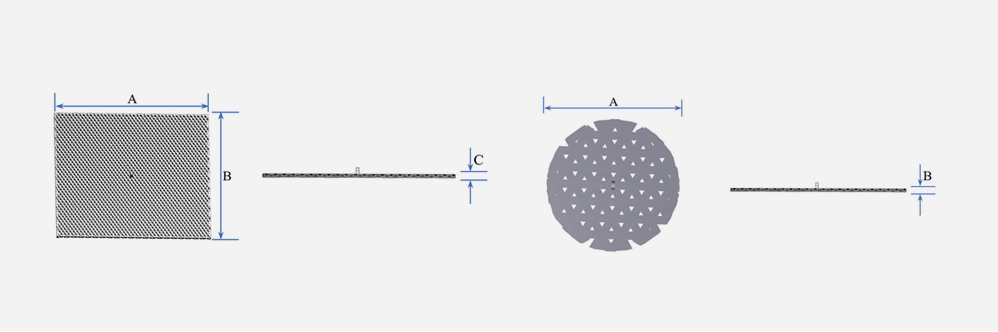
| Width(A) | Depth(B) | Height(C) |
|---|---|---|
| 10mm | 10mm | 0.5mm |
| 10mm | 10mm | 1.0mm |
| 20mm | 20mm | 0.5mm |
| 20mm | 20mm | 1.0mm |
| 40mm | 40mm | 0.8mm |
| 50mm | 50mm | 0.8mm |
| 50mm | 50mm | 1.0mm |
| Diameter(A) | Depth(B) |
|---|---|
| 10mm | 0.5mm |
| 10mm | 1.0mm |
| 20mm | 0.5mm |
| 20mm | 1.0mm |
| 40mm | 0.8mm |
| 50mm | 0.8mm |
| 50mm | 1.0mm |