2023
05Siheung Campus Completion Ceremony
0310th Anniversary Workshop
02Johnson & Johnson Medical Korea Ltd.Signed a contract to supply patient-specific implants.


2022
10Signed MOU with Lee Yeon Pharmaceutical for cooperation in the development of complex hemostatic medical device
09Signed a contract with Neopharm for the supply of wound covering materials
06Johnson & Johnson Mentor Worldwide LLC. Signed a Strategic Joint Research Agreement to Develop 3D Bioprinting Scaffold Technology for Tissue Reconstruction
01Completed the construction of our commercial cell bank for human-derived cells
2021
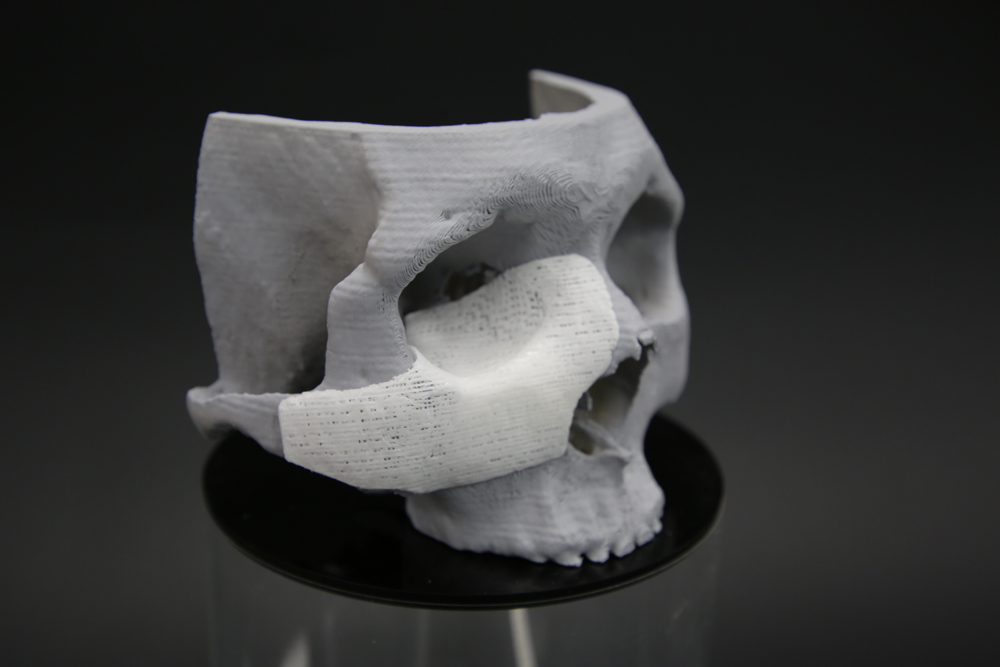
08Successful clinical application of 3D-printed biodegradable scaffold for microtia
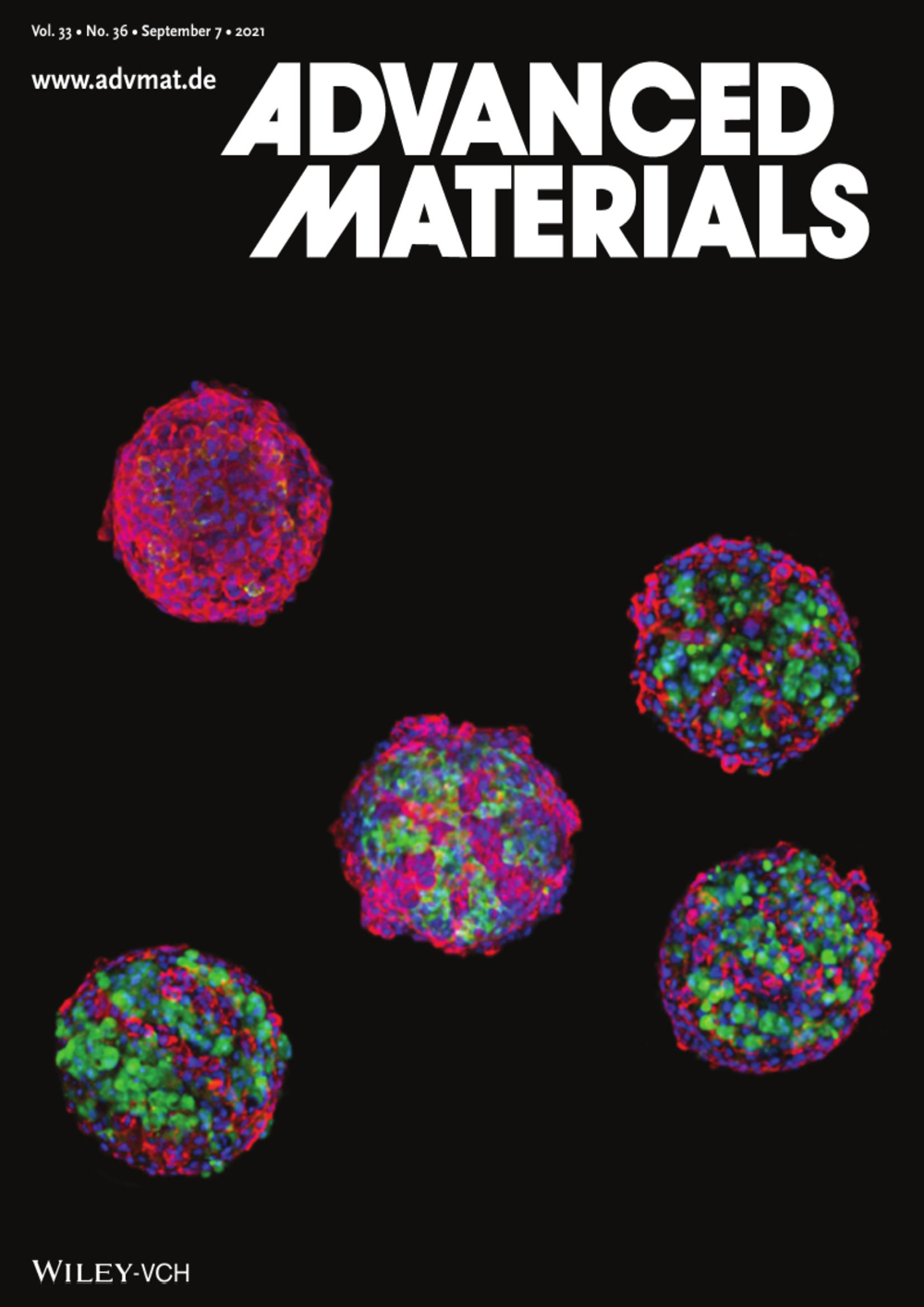
07Research on development and in-vivo transplantation of 3D-bioprinted liver organoids published in top scientific journal, Advanced Materials
02B.Braun Korea obtained product license from Ministry of Food and Drug Safety for biodegradable craniofacial implants and commenced sales activities in Korea

2020
12Received IND approval for Korea's first biodegradable artificial support for ear reconstruction
09'Induced pluripotent stem cell-based cardiomyocytes' sales license obtained
02Johnson & Johnson Medical Ethicon Signed Joint Research Agreement to Develop 3D Bioprinting Scaffold Technology for Tissue Reconstruction
02Successful development of 'liver organoids' published in top scientific journal, Small



2019
07Development of myocardial infarction cell therapy using 3D cell printing technology published in top scientific journal, Nature



2018
11Listed on KOSDAQ
09Accreditation as a Quality management system - ISO 9001
03Accreditation as a Quality management system - ISO 13485
021st T&R Biofab Symposium : 3D Bioprinting Pioneer Committee

2017
04Launched deCelluid™(Bioink)
02Establishment of Research Complex for the Institute for Industry-Academy Research Foundation (Pusan National University Hospital)
01Launched TnR Scaffold for research
2016
06Opening of Open Innovation Center (POSTECH)
04Investment promotion (125K USD, HB Investment)
03Opening of 3D Printing-based Clinical Center (Seoul St. Mary’s Hospital)
03Investment promotion (292K, HUGEL Inc., etc.)
02Investment promotion (167K USD, Korea Investment Partners)


2015
12Investment promotion
(167K USD, Korea Development Bank)
10Moved to a new headquarters (Smart Hub of University-Industry Convergence HQ #540, 237 Sankidaehak-ro, Siheung-si, Gyeonggi-do, Korea)
2014
12Investment promotion
(167K USD, Korea Investment Partners)
09Patent transfer agreement with Industry-Academy Foundation of POSTECH
09First clinical application in Seoul St. Mary’s Hospital
08Accreditation as a Research and Development Center
08Individual customized item approval
07Initial item approval (special material skull molding material, grade 4)
05GMP certification (Level 4 medical devices)
05Accreditation as a Venture Company

2013
03Establishment of T&R Biofab (Starting capital: 41K USD) (Headquarters: Techno Innovation Park #407, 237 Sankidaehak-ro, Siheung-si, Gyeonggi-do, Korea)